Important note: Minnesota Clocks & Watches is grateful to the Minnesota Pollution Control Agency (MPCA) who reviewed this article and provided valuable input. While Minnesota Clocks & Watches (MC&W) believes all information presented in this article to be correct, mercury is a hazardous substance and MC&W does not warrant that the information and procedures described here eliminate risks of mercury spillage, exposure, or health effects. Proceed at your own risk.
One of the more startling aspects of high-end antique clocks is the use of mercury in some pendulums. Why was this done? It was a way of improving clock accuracy by compensating for the effects of temperature change on the clock.
Pendulum Physics
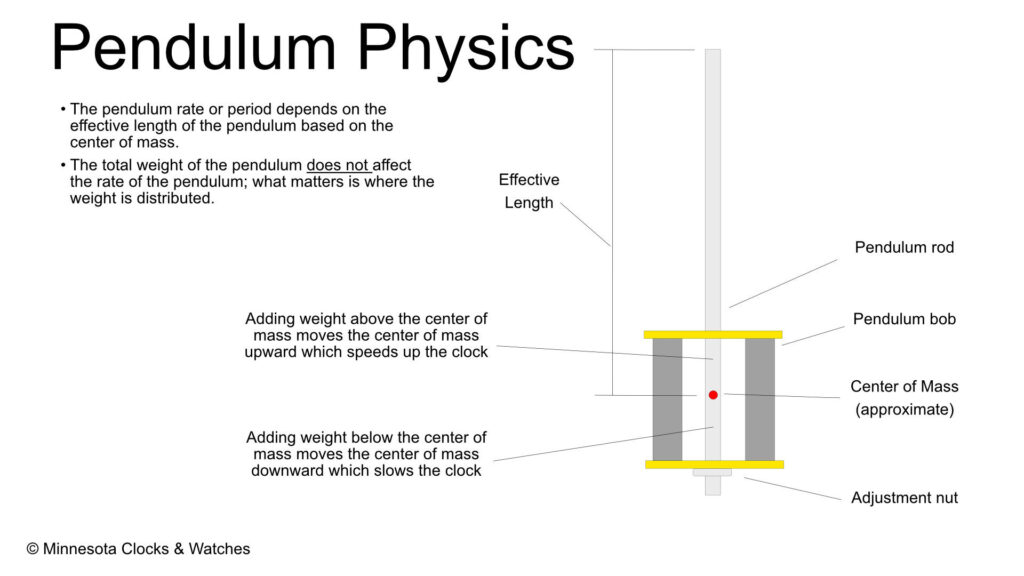
The rate of a clock is determined primarily by the effective length of the pendulum. This is not a simple measurement, as the weight distribution up and down the pendulum affects the rate. Note that the weight of a pendulum has no effect on the rate of the pendulum; rather it is the relative weight distribution that affects the rate of a pendulum. If you add weight above the center of mass, the speed of the pendulum will actually increase, because you are raising the center of mass* of the pendulum.
Compensation
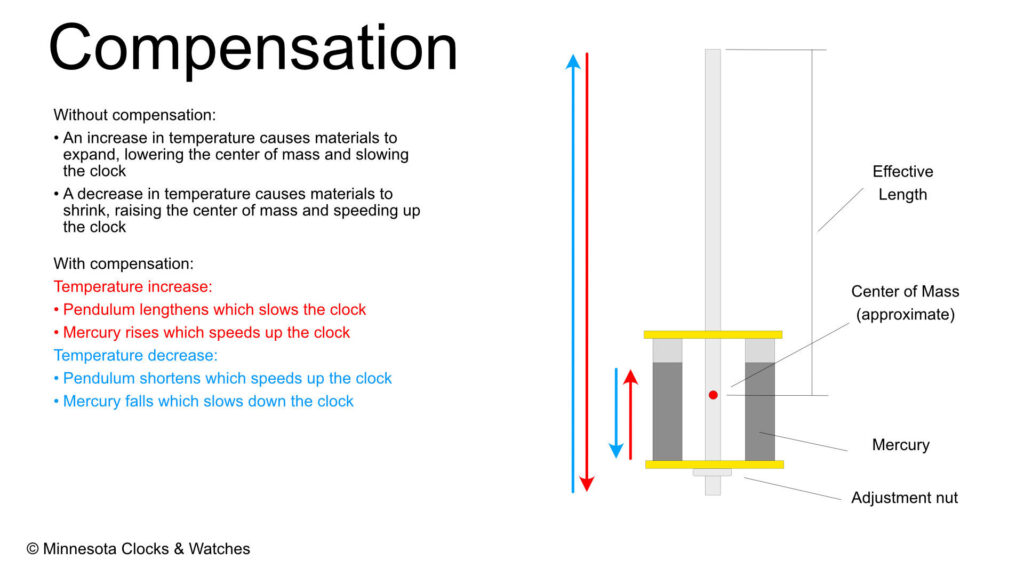
Pendulums are remarkably consistent devices, which makes them ideal for timekeeping. A pendulum is only as good as its materials, though. Nearly every material expands when temperature rises and shrinks when temperature falls. In a clock, this means that in the summer a clock will run slower as the pendulum has expanded due to the temperature increase. This lengthens the pendulum, which lowers the center of mass and slows the clock. Conversely in the winter, the pendulum shrinks, which raises the center of mass, speeding up the clock.
This behavior cannot be overcome – it is the nature of materials. It can be compensated for, which is where mercury comes in.

Mercury, like all other materials, expands and contracts with temperature changes. In a compensating mercurial pendulum, the mercury in the jars rises with a temperature increase, raising the center of mass of the pendulum, which compensates for the lengthened pendulum rod which lowered the center of mass.
There are several methods to compensate for temperature, but one advantage of mercury is that it is adjustable by the user. To increase compensation (the clock still runs slower in the summer and faster in the winter), you can add mercury. If your clock runs faster in the summer and slower in the winter, your pendulum is over compensated – it’s adjusting too much – you can remove some mercury.
The main disadvantage of mercury is it can be a health hazard.
Mercury Toxicity
This is an issue that has three components: the science of the health risks of mercury exposure, legal factors, and personal comfort level. I will endeavor to keep these factors separate and be clear about what is factual and what is an opinion.
Let’s start with the science. Mercury, like many other heavy metals is toxic. The degree of toxicity depends on how much contact and, more importantly, the form of the mercury. There are three main categories of mercury compounds: organic mercury, inorganic mercury, and elemental or metallic mercury. Elemental mercury – the kind used in clock pendulums – is by far the least toxic of these three kinds, and is not readily absorbed by the body.
Wikipedia states the following:
Quicksilver (liquid metallic mercury) is poorly absorbed by ingestion and skin contact. Its vapor is the most hazardous form. Animal data indicate less than 0.01% of ingested mercury is absorbed through the intact gastrointestinal tract, though it may not be true for individuals suffering from ileus. Cases of systemic toxicity from accidental swallowing are rare, and attempted suicide via intravenous injection does not appear to result in systemic toxicity…
In the interest of keeping this article on task, I’m going to provide some references below that go into much more detail, and we will jump right to the punchline: As long as the mercury stays inside your clock, you are just fine. In fact, mercury probably doesn’t even make the top 10 list of most hazardous substances in your home: bleach, drain cleaner, gasoline, propane, and many other household chemicals are much more likely to cause injury or death than mercury exposure, as the only risk of mercury is a spill, which is an event that you will be aware of and can take action to prevent a health risk.
Mercury Vapor
Metallic mercury does evaporate slowly at room temperature, but as long as there is a cover over the mercury, the health risk from unspilled mercury is low, as there is little air exchange between the covered mercury vial and the room. Whatever very small amount of mercury vapor that may escape from the covered mercury vials in your clock will be exchanged with the outside air (which already contains a small amount of mercury from industrial pollution and natural geological activity) through open doors and windows and the air leakage of your home (note that mercury vapor is heavy and accumulates near the floor, so if small children are present, that may change your risk calculation due to their greater sensitivity to mercury exposure and the amount of time spent on the floor).
The greater risk of mercury exposure comes when mercury is spilled and spread over a large area. Consider a glass of water. If the water is in a glass, only the surface of the water can evaporate. All the water below the topmost layer of water is held captive by the water above it, and so it takes days or a week or more for a glass of water to evaporate. If you take that water and pour it on the floor, now the surface area of water exposed to the air is many times greater, which means the same volume of water will evaporate quickly – in just a couple hours.

Mercury is the same. Mercury vials in a clock are tall and slender and have a very small surface area, and every mercury pendulum has at least a metal cap; others are entirely sealed or corked. Even with the standard metal cap, the mercury has very little exposure to the outside environment, and therefore will have almost zero evaporation. If mercury is spilled, now the surface area of the mercury is much greater and cleanup is a concern, as mercury droplets spread and attach themselves to fabrics and other porous surfaces. This lingering mercury is a significant health hazard, especially to children, and a thorough cleanup by a qualified organization is essential.

Here is where we leave the concrete science behind and enter into the legal realm and the realm of personal comfort level.
Cleaning Up a Spill
In Minnesota, the MPCA is the governing body that is in charge of handling mercury spills and has released a guide for mercury.
Households are encouraged to notify Minnesota’s free 24-hour hazardous incident reporting system, the Minnesota Duty Officer, for any except de minimus mercury spills (such as a single broken compact fluorescent lamp).
Businesses, including clock repair providers, are required to notify the state of any mercury spills.
MPCA
Large spills such as an entire mercury vial spilling would be considered to be a mandatory reporting event and require professional cleanup. Smaller spills are up to the owner to determine the response. The EPA site mentioned in the Additional Resources section below contains procedures to cleanup small mercury spills, including using powdered sulfur, which binds with the mercury, preventing vaporization.
In Minnesota, Mercury is a regulated substance. It is not illegal to own mercury in Minnesota, however Minnesota does ban the sale and distribution of mercury in clocks and clock components. According to the MPCA:
Technically, Minnesota law also prohibits the use of mercury-containing weights [including pendulums], however the MPCA does not currently and has no plans to enforce that use provision against citizens continuing to use historic artifacts such as clocks.
You may find guidance from the MPCA regarding mercury item sales and restrictions in MPCA Factsheet #w-hw4-26.
Thus ends the legal elements; what remains is personal comfort level.
Is mercury worth the risk?
While unspilled mercury is not a significant health concern, spilled mercury is. The good news is you will know when this happens and can take action to initiate cleanup before any health risks from exposure can occur. Before choosing to keep mercury in your clock, you may want to see if mercury spills are covered by your homeowners insurance.
Many people feel the risks of a mercury spill outweigh the historicity and coolness of a clock with an intact mercury pendulum. That is very understandable, and many people remove the mercury from their pendulum and replace it with steel or lead shot. Your clock will run just fine like this, though with less effective compensation.
My Perspective
My personal comfort level has evolved after doing research on this issue. My first mercury pendulum clock involved an accidental spill of a very tiny amount of mercury – the size of a sesame seed or so, which launched the research project and in a moment of concern even brought me to the doctor for a mercury test (well in the normal range). After looking up the OSHA exposure limit for mercury and doing some math, it became clear that it would take much more mercury to be a problem, and I have focused my concern on making sure the clock and its environment are sound and the mercury is unlikely to spill.
Lowering the Risk

If you choose to have mercury in your clock, there are three factors that I believe are important to greatly reducing your risk of an incident:
- Anchor your clock to a wall. I don’t care if you have to drill a hole in your clock to do it; I believe this is critically important to prevent someone from bumping into your clock and tipping it over. One way of minimizing damage to your clock is to use a metal angle bracket mounted on the top of your clock like I demonstrated in my Setting Up A Jeweler’s Regulator video.
- Keep the clock door locked, if equipped, or latched at all times to keep kids and other curious people out.
- Have the clock inspected periodically to make sure the case, movement mounts, suspension spring, and other components are solid. Pendulum suspension springs do occasionally break, but inspecting when the clock is serviced every 10 years or so should make sure you stay ahead of any metal fatigue.
Transporting Mercury
Mercury can be transported in a personally-owned vehicle legally. Ironically, you will have a very difficult time finding any company that would be willing to transport it for you due to the hazmat requirements, and due to the potential for mercury to weaken aluminum, mercury is banned from nearly all airplanes.
Mercury should always be stored or transported in plastic or glass containers – never metal – as mercury reacts with many kinds of metal, especially aluminum which is used in cookware. Most steels do not react with mercury, however stainless steel does (some kitchen sinks and kitchenware. Also, as not all steel containers are liquid-tight, a steel container should only be used as secondary containment.

Here is my method of transporting mercury – a glass container with packaging material inside a steel paint can with an interior coating. The packaging material is vermiculite. This setup provides two layers of containment plus some shock protection. The vermiculite packs down and prevents the jar from tipping inside the outer can.
Working With Mercury

Additional caution: The cost of cleanup of a mercury spill can be significant and may not be covered under your homeowner’s insurance. Use extreme care and perform any transferring of mercury in an area where cleanup is easier – e.g. outside on your driveway. In the event of any spilled mercury, isolate the area to prevent mercury contamination from being spread by foot traffic and contact the proper authorities to initiate cleanup.
When disassembling a mercury pendulum clock, if the pendulum looks like the photo above, use one hand to hold the mercury vial and lift up the metal cap with the other hand and carefully lift out the mercury jar. It may or may not have a seal of some kind. If it is tightly sealed, you may be able to place the filled mercury vials into a larger container as long as you can pack your container so that the vials remain vertical. If the vial is not sealed or is only marginally sealed, you need to pour the mercury into a sealed plastic or glass container and then pack as I described above.
When assembling a mercury pendulum clock, leave the mercury until last. Get the clock case assembled and secured to a stud in the wall, inspect then mount the movement (hire someone to do this; this is a great time to have the movement serviced), and hang the pendulum frame without mercury vials on the clock. Wind the clock, set it in beat, and let it run for at least 24 hours to make sure the movement doesn’t require adjustments.

Only after everything else is good, refill the mercury vials from your transport container. It is extremely important that you have a glass or plastic tray with side walls under where you are filling the vials. Spilled mercury droplets have a tendency to shoot off from where they landed, and a glass or plastic tray will contain any mercury droplets. DO NOT USE AN ALUMINUM TRAY – mercury will react with the aluminum.
Do this in a well-ventilated area – open windows and use fans if possible. Your exposure during the few minutes this takes will be low, but this is an easy precaution.


Pouring mercury from a vial into a larger container is not dangerous, but do not try to pour from a large container back into your mercury vials. Instead, get a pipet and use it to slowly transfer the mercury into the vials. This gives you much greater control and you are much less likely to have a spill. When pipetting, keep the pipet vertical, don’t overfill it, and move slowly otherwise the weight of the mercury could overcome the vacuum and drip out. Tipping the vial over the larger jar while transferring ensure any drips from the pipet just fall back into the larger container.

If possible, try to seal the vials. This eliminates any vapor leakage that may escape the original metal cap. Here is a section of a cork I cut to fit in the vial. Because the cork is thin, there is a void in the cork where mercury could escape, so I have layered two layers of plastic wrap under the cork to get a better seal.

Here is the finished product – a New Haven Regulator No. 4 standing at at whopping 122″ tall. Thanks for riding along.

Additional Resources
Minnesota Pollution Control Agency Spill Guidance
Center for Disease Control on mercury toxicity
Cody’s Lab on YouTube (search for mercury videos)
*The term “center of oscillation” is more correct than “center of mass”, but center of mass is used because the concept is more familiar.

2 replies on “Handling Mercury In Clocks”
I have inherited a Waterbury 61 with a jewelers mercury two tube pendulum. On the pendulum are two dials. The lower one has a pointer and fine graduation lines. The upper dial has “knobs”. Both of these can be turned right or left
What is each one used for?
The lower one with the pointer is the coarse adjustment that raises the entire mercury holding apparatus; the upper knob with the bumps is the fine adjustment that only raises and lowers that nut.